Tissue organization and cell ultrastructure in the roots of three Arabidopsis species grown at different zinc concentrations
Abstract
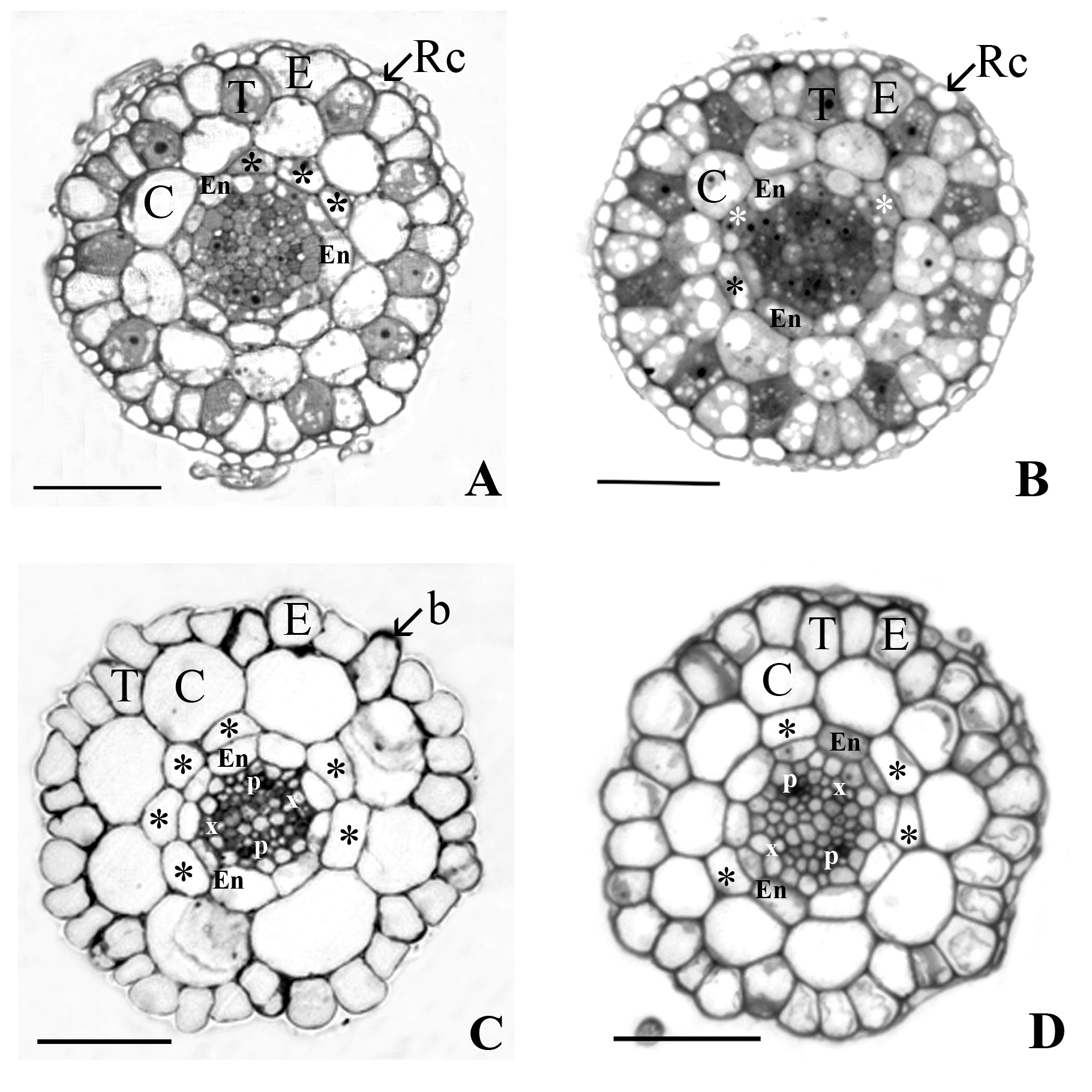
The model plant Arabidopsis thaliana is known to be heavy metal-sensitive in contrast to its relative species A. arenosa and A. halleri classified as pseudometallophytes. Quantitative differences in primary root anatomy previously found between A. thaliana and the non-metallicolous (NM) and metallicolous (M) populations of the non-model Arabidopsis species necessitated further research at cellular and ultrastructural levels. Seedlings of A. thaliana, ecotype Columbia and a natural population Ratkovo, the NM and M populations of A. arenosa and A. halleri were grown on agar medium containing 10 µM (control) and 1000 µM Zn2+ for 5 days. Light microscopy confirmed the higher number of cells in the endodermal, cortical and epidermal layers and a higher incidence of additional cell tiers, the so-called middle cortex (MC) in the tolerant genotypes. Such differences were present in untreated plants and even more pronounced in plants exposed to excess of zinc (Zn). Electron microscopy of the root tissues at comparable distances from the root tip showed Casparian bands only in the radial cell walls of endodermis of A. halleri M population originating from severely (Cu, Cd and Pb) contaminated site. Casparian bands were not differentiated yet in the roots of the other species and populations, and they were not formed in the cell walls between endodermis and MC cells. In the apical cytoplasm of trichoblast bulges, autophagic vacuoles were found only in the sensitive A. thaliana and small vacuoles in the other genotypes. The enhanced concentration of Zn confirmed the higher metal sensitivity of the model species and did not substantially disturb the root cell ultrastructure of the tolerant Arabidopsis species.
References
Azzarello A., Pandolfia C., Giordano C., Rossia M., Mugnaia S., Mancuso S. 2012. Ultramorphological and physiological modifications induced by high zinc levels in Paulownia tomentosa. Env. Exp. Bot. 81: 11–17.
Banásová V., Horak O., Čiamporová M., Nadubinská M., Lichtscheidl I. 2006. The vegetation of metalliferous and non-metalliferous grasslands in two former mine regions in Central Slovakia. Biologia, Bratislava 61: 433–439.
Bassham D.C., Laporte M., Marty F., Moriyasu Y., Ohsumi Y., Olsen L.J., Yoshimoto K. 2006. Autophagy in development and stress responses of plants. Autophagy 2: 2–11.
Baum S.F, Dubrovsky J.G, Rost T.L. 2002. Apical organization and maturation of the cortex and vascular cylinder in Arabidopsis thaliana (Brassicaceae) roots. Am. J. Bot. 89: 908–920.
Broadley M.R., White P.J., Hammond J.P., Zelko I., Lux A. 2007. Zinc in plants. New Phytol. 173: 677–702.
Clauss M.J., Koch M.A. 2006. Poorly known relatives of Arabidopsis thaliana. Trends Plant Sci. 11: 421–468.
Dechamps C., Elvinger N., Meerts P., Lefèbvre C., Escarre J., Colling G., Noret N. 2010. Life history traits of the pseudometallophyte Thlaspi caerulescens in natural populations from Northern Europe. Plant Biol. (Stuttgart) Suppl. 1: 125–135. doi: 10.1111/j.1438-8677.2010.00387.x.
Dolan L., Janmaat K., Willemsen V., Linstead P., Poethig S., Roberts K., Scheres B. 1993. Cellular organization of the Arabidopsis thaliana root. Development 119: 71–84.
Galway M.E., Heckman J.W. Jr., Schiefelbein J.W. 1997. Growth and ultrastructure of Arabidopsis root hairs: the rhd3 mutation alters vacuole enlargement and tip growth. Planta 201: 209–218.
Hayashi Y., Yamada K., Shimada T., Matsushima R., Nishizawa N.K., Nishimura M., Hara‑Nishimura I. 2001. A proteinase-storing body that prepares for cell death or stresses in the epidermal cells of Arabidopsis. Plant Cell Physiol. 42: 894–899.
Kenderešová L., Staňová A., Pavlovkin J., Ďurišová E., Nadubinská M., Čiamporová M., Ovečka M. 2012. Early Zn2+-induced effects on membrane potential account for primary heavy metal susceptibility in tolerant and sensitive Arabidopsis species. Ann. Bot. 110: 445–459.
Kupidlowska E. 2001. Changes in cell ultrastructure and morphology of Arabidopsis thaliana roots after coumarins treatment. Acta Soc. Bot. Polon. 70:187–198.
Lux A., Martinka M., Vaculík M., White P.J. 2011. Root responses to cadmium in the rhizosphere: A review. J. Exp. Bot. 62: 21–37.
Martinka M., Dolan L., Pernas M, Abe J., Lux A. 2012. Endodermal cell-cell contact is required for the spatial control of Casparian band development in Arabidopsis thaliana. Ann. Bot. 110: 361–371.
Martinka M., Vaculík M., Lux A. 2014. Plant cell responses to cadmium and zinc. In: Nick P., Opatrný Z. (eds), Applied plant cell biology. Cellular tools and approaches for plant biotechnology: 209–246. Springer-Verlag, Berlin, Heidelberg.
Richard O., Pineau C., Loubet S., Chalies C., Vile D., Marquès L., Berthomieu P. 2011. Diversity of the response to Zn within the Arabidopsis thaliana species revealed a low contribution of Zn translocation to Zn tolerance and a new role for Zn in lateral root development. Plant Cell Environ. 34: 1065–1078.
Roosens N.H.C.J., Willems G., Saumitou-Laprade P. 2008. Using Arabidopsis to explore zinc tolerance and hyperaccumulation. Trends Plant Sci. 13: 208–215.
Scheres B., Wolkenfeelt H., Willemsen V., Terlouw M., Lawson E., Dean C., Weisbeek P. 1994. Embryonic origin of the Arabidopsis primary root and root initials. Development 120: 2475–2487.
Staňová A., Banásová V., Čiamporová M. 2010. Strategies of three Arabidopsis species under heavy metal excess in the soil In: Benčaťová B., Benčať T. (eds), Flóra a vegetácia Oravy. Bulletin Slovenskej botanickej spoločnosti 32 (Suppl. 2): 237–246. (in Slovak)
Staňová A., Ďurišová E., Banásová V., Gurinová E., Nadubinská M., Kenderešová L., Ovečka M., Čiamporová M. 2012. Root system morphology and primary root anatomy in natural non-metallicolous and metallicolous populations of three Arabidopsis species differing in heavy metal tolerance. Biologia, Bratislava 67: 505–516.
Turisová I., Štrba T., Aschenbrenner Š., Andráš P. 2013. Arabidopsis arenosa (L.) Law. on metalliferous and non-metalliferous sites in central Slovakia. Bull. Environ. Contam. Toxicol. 91: 469–474.
Vaculík M., Konlechner C., Langer I., Adlassnig W., Puschenreiter M., Lux L., Hauser M-T. 2012. Root anatomy and element distribution vary between two Salix caprea isolates with different Cd accumulation capacities. Environ. Pollut. 163: 117–126.
Verbelen J-P., De Cnodder T., Jie Le, Vissenberg K., Baluška F. 2006. The root apex of Arabidopsis thaliana consists of four distinct zones of growth activities. Meristematic zone, transition zone, fast elongation zone and growth terminating zone. Plant Signal. Behav. 1: 296–304.
Zelko I., Lux A., Czibula K. 2008. Difference in the root structure of hyperaccumulator Thlaspi caerulescens and non-hyperaccumulator Thlaspi arvense. Int. J. Environ. Pollut. 3: 123–132.
Zhu T., Lucas W.J., Rost T.L. 1998. Directional cell-to-cell communication in the Arabidopsis root apical meristem I. An ultrastructural and functional analysis. Protoplasma 203: 35–47.

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
The journal is licensed by Creative Commons under BY-NC-ND license. You are welcome and free to share (copy and redistribute the material in any medium or format) all the published materials. You may not use the material for commercial purposes. You must give appropriate credit to all published materials.
The journal allow the author(s) to hold the copyrights and to retain publishing rights without any restrictions. This is also indicated at the bottom of each article.





